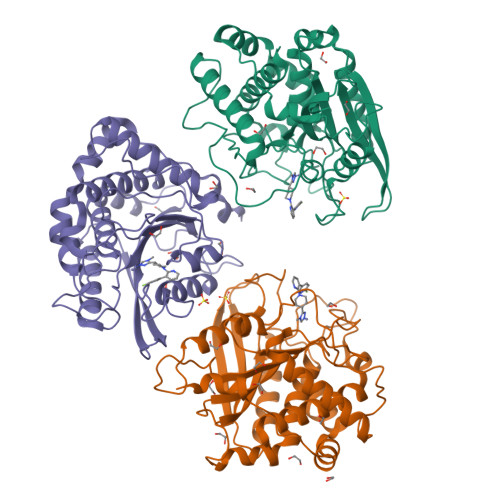
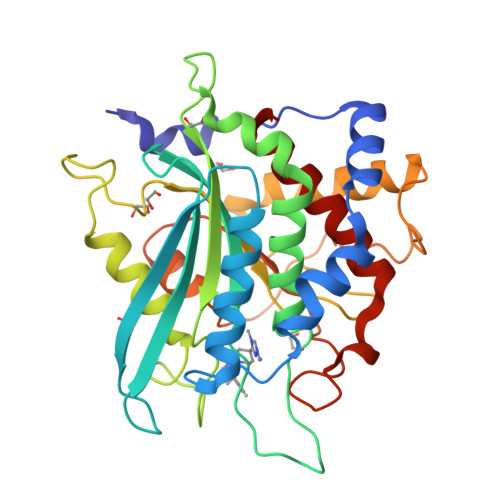
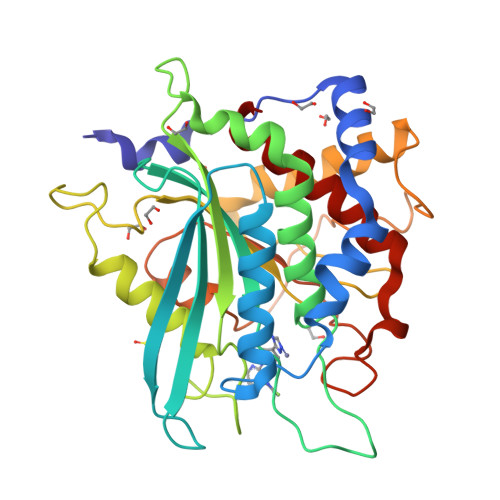
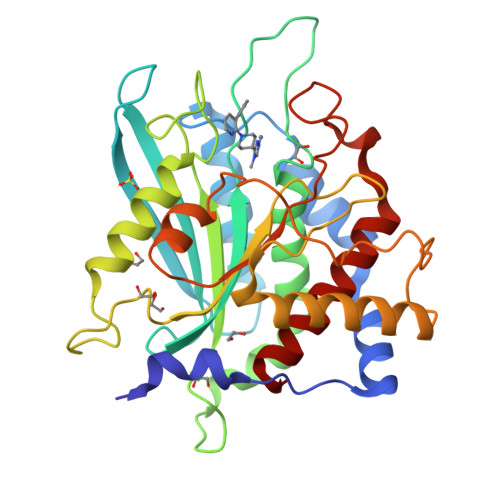
The structure of the human glutaminyl cyclase-SEN177 complex indicates routes for developing new potent inhibitors as possible agents for the treatment of neurological disorders.
Pozzi, C., Di Pisa, F., Benvenuti, M., Mangani, S.(2018) J Biol Inorg Chem 23: 1219-1226
- PubMed: 30132075
- DOI: https://doi.org/10.1007/s00775-018-1605-1
- Primary Citation of Related Structures:
6GBX - PubMed Abstract:
Recent evidence links the role of human glutaminyl cyclase (hQC) to the amyloidogenic process involved in Alzheimer's disease (AD). hQC is a zinc enzyme present in neuronal tissue and its activity is responsible for the cyclization of N-terminal Gln or Glu β-amyloid peptides, leading to N-pyroglutamic acid peptides (pE-Aβ) that is probably a crucial event in the initiation and progress of the disease. Indeed, pE-containing peptides exhibit an elevated neurotoxicity and a tendency to aggregate. These observations render hQC inhibition an attractive strategy for developing new molecules active against AD. We present here the crystal structure of hQC in complex with SEN177, a newly designed molecule. The SEN177-binding mode to hQC differs from that of the known hQC inhibitors. SEN177 K i on hQC is 20 nM, comparable or better than that of the most potent known hQC inhibitors PBD150 and PQ912. In addition, SEN177 already demonstrated relevant pharmacological properties in in vivo models of Huntington's disease. All these properties make SEN177 an important scaffold for developing molecules acting on AD and related diseases.
Organizational Affiliation:
Department of Biotechnology, Chemistry and Pharmacy, Department of Excellence 2018-2022, University of Siena, via Aldo Moro, 2, 53100, Siena, SI, Italy.